Achieving Cell-to-cell Communication

Uniform Micro Surface Geometry and Maximized BIC for Accelerated Osseointegration

Long-lasting surface achieved with special technology, eliminating the need for additional processes such as UV and Plasma in the clinic

Unlike other implant surfaces, does not use titanium oxide but rather a biocompatible material that is sandblasted for a human-friendly surface

rhBMP-2 optimized
for implant placement

Strong surface stability that withstands up to 120Ncm of force

Maximized contact area between alveolar bone and implant
Key Features
01Accelerating Osseointegration by Forming Uniform Macropores and Micropores through Precise Acid Etching
Comparison of SEM Images of SLA Surface Treated Implants Sold Domestically
- Surface treatment images observed in electron microscopy images magnified 5,000 times from the upper part of the implant
- In the SLA surface oxide layer modeling process of Company A, B, and C products, the deeper areas show weak penetration, resulting in non-uniform oxide layers and visible initial roughness, with some areas showing incomplete modeling.
- In contrast, Cowellmedi INNO implants demonstrate uniformity, consistency, and minimal variation (roughness) in the SLA surface oxide layer modeling process.This indicates that INNO SLA oxide layer modeling has a relatively much higher degree of completeness.
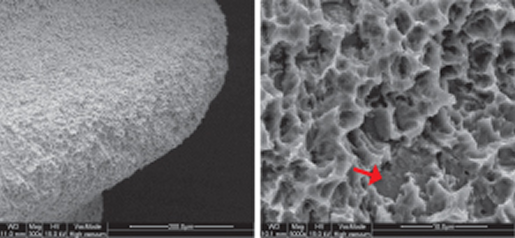
A Implant
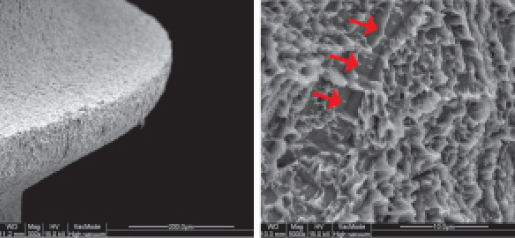
B Implant
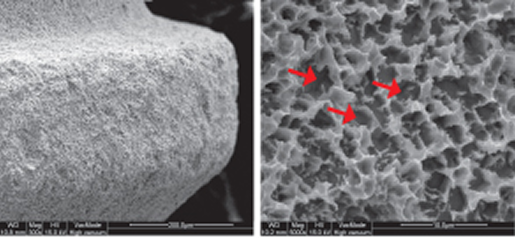
C Implant
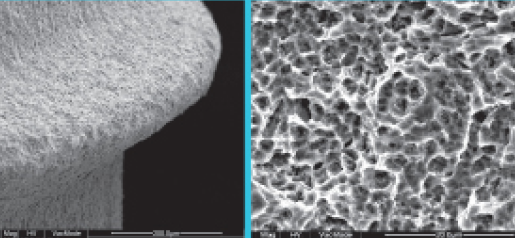
Cowellmedi
Comparison of Domestic SLA Surface Treatment 3D Roughness Measurement Results
- The average surface roughness value ranges from 1.07 to 3.11μm, and in the case of Cowellmedi INNO implants, the surface roughness is evenly distributed across the unit area, with the calculated average roughness value being 1.92μm.
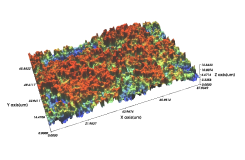
Cowellmedi
- Sdr(%) : 245
- Sa(㎛) : 1.88
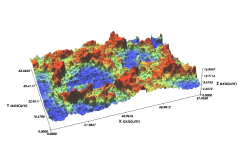
A Implant
- Sdr(%) : 219
- Sa(㎛) : 2.04
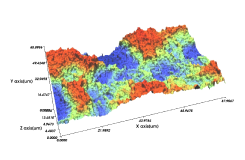
B Implant
- Sdr(%) : 215.33
- Sa(㎛) : 2.61
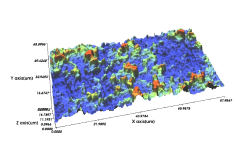
C Implant
- Sdr(%) : 239
- Sa(㎛) : 1.79
Surface Structure Strength & Micro-fit Survival Rate Comparison
- Cowellmedi INNO implants maintain stable Micro-fit structures even under high torque conditions of 40N–80N.
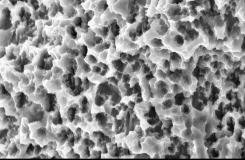
Cowellmedi

A Implant
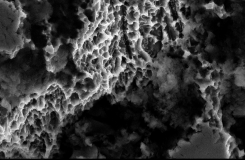
B Implant
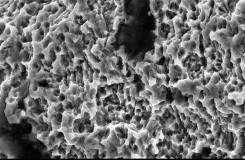
C Implant
02creased Surface Activation
Contact Angle Measurement Results for Physiological Saline
- Increased energy facilitates better attachment of bone cells, enabling faster osseointegration
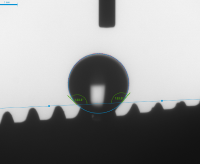
Other Implant

Cowellmedi
- Capillary Action Promoting Blood Infiltration in Actual Clinical Practice
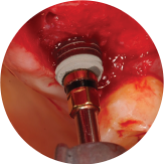
Blood Friendly
03Proven Safety through Perfect Cleaning with Automated Systems
Surface Component Inspection (X-ray Photoelectron Spectroscopy, XPS) Comparison
- Quantitative analysis of surface components shows that all products have approximately 30% carbon, 47% oxygen, 16% titanium, and 4% silicon
- Cowellmedi INNO SLA consists only of carbon (C1s), oxygen (O1s), and titanium (Ti2p) components, with the main component of the alkaline cleaning solution, sodium hydroxide (NaOH), combining with silicon (Si) to form water-soluble Na2SiO2(OH)2·4H2O (water glass), removing other components

| Sample | C1s | O1s | Ti2p | Si2p | N1s |
|---|---|---|---|---|---|
| Company A | 34.12 | 45.05 | 15.11 | 5.24 | 99.99% |
| Company B | 31.84 | 46.49 | 15.22 | 4.87 | 99.99% |
| Company C | 32.19 | 47.58 | 17.58 | 2.65 | 100% |
| Cowellmedi | 15.92 | 58.65 | 25.43 | N.D | 95.61% |
Comparison of Extracts Using Combustion Ion Chromatography
- Similar ions were detected in all products, but they are safe for the human body due to their harmless nature and quantity
- In the case of Cowellmedi INNO SLA, no components other than NO3- were detected. SO42- and Cl- ions, which are used for heated acid etching by alkaline cleaning, form water-soluble salts Na2SO4 and NaCl and are completely removed.
- No components that hinder bone regeneration were detected in both surface and extract components, indicating that the cleaning process is very well executed.Extract from Dr. Lee Dae-hee's research paper (published in June and August 2012 in Dentopline / KDM) -
| Sam ple |
F- | CI- | NO2- | SO42- | Br- | NO3- | PO43- |
|---|---|---|---|---|---|---|---|
| Company A | N.D | 0.024 | 0.027 | 0.002 | N.D | 0.031 | N.D |
| Company B | N.D | 0.027 | 0.019 | 0.002 | N.D | 0.030 | N.D |
| Company C | N.D | 0.071 | 0.020 | 0.002 | N.D | 0.023 | N.D |
| Cowellmedi | N.D | N.D | N.D | N.D | N.D | N.D | N.D |